Abstract
§ s hared first authorship; §§ s hared last authorship
Primary Central Nervous System Lymphoma (PCNSL) is a rare and aggressive non-Hodgkin lymphoma with limited treatment options and severe prognosis. It may display copy number abnormalities of the 9p24.1 locus harboring the programmed cell death protein ligand 1 (PD-L1) gene. Moreover, tumor lesions often overexpress programmed cell death protein 1 (PD-1) as well as its ligands (PD-L1/PD-L2). Soluble forms of these molecules have been demonstrated in serum and plasma, though the biological and clinical significance of these soluble checkpoint proteins is yet unclear. Furthermore, their presence in the cerebrospinal fluid (CSF), particularly in malignant diseases, is largely unexplored. Thus, the aim of our study was to measure the occurrence of soluble PD-1 (sPD-1) in the CSF of PCNSL patients.
We measured sPD-1 levels in pretherapeutic plasma and CSF samples from 11 patients with histopathologically confirmed PCNSL of diffuse large B-cell type (DLBCL) and five patients in which the histopathological examination did not confirm the initial clinical and imaging-based suspicion of PCNSL diagnosis (non-PCNSL). All blood and CSF samples were obtained prior to neurosurgical biopsy. Of these five patients, four had reactive non-malignant lesions and one a glioblastoma. Six CSF samples routinely screened for non-malignant neurological disease (e.g. sclerosis) were included as an additional non-PCNSL control group (neuro-screen samples). All samples were handled identically prior to analysis. Soluble PD-1 was measured using an in-house time-resolved immunofluorometric assay (TRIFMA) as previously reported by our group (Mortensen JB et al. Eur J Haematol. 2021 Jul;107:81-91). All samples were analyzed in duplicates. The TRIFMA was validated for freeze/thaw interference and recovery.
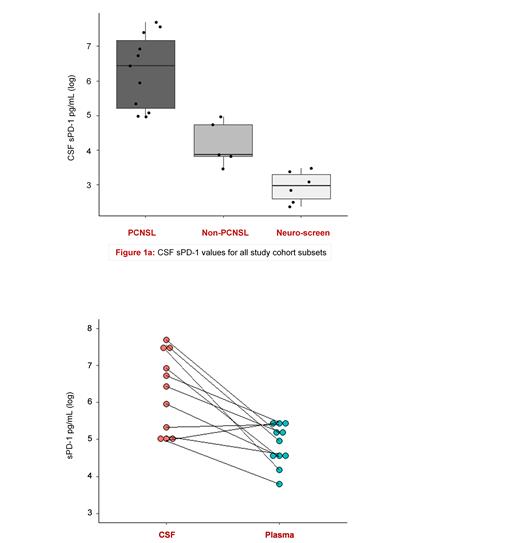
We found that pretherapeutic CSF sPD-1 levels in PCNSL patients were approximately 10 to 30-fold higher than those of non-PCNSL and neuro-screen patients (p<0,001). The median CSF sPD-1 level for PCNSL was 628 pg/mL, while the corresponding values for the non-PCNSL and neuro-screen subgroups were 48 pg/mL and 19 pg/mL, respectively. Fig. 1a shows the sPD-1 values measured in the three different subsets (PCNSL, non-PCNSL, neuro-screen) of the study cohort. Three PCSNL patients had particularly high sPD-1 values (range: 1642-2194 pg/mL) in their pretherapeutic CSF samples. Interestingly, these three patients all displayed 'high-risk' molecular features, i.e., one case of double-overexpression of bcl-6 and c-Myc and the only two cases of triple-overexpression of bcl-2, bcl-6 and c-Myc. In all 11 PCNSL patients, the DLBCL histology had an activated B-cell like (ABC)/non-germinal center B-cell like (non-GCB) signature. Plasma samples were only available for the PCNSL and non-PCNSL subsets. The median plasma sPD-1 value was 142 pg/mL and 124 pg/mL, respectively. As opposed to non-PCNSL, the CSF sPD-1 levels of PCNSL patients were higher than the paired plasma values (p<0.001; Fig.1b). TRIFMA based measurement of sPD-L1/2 in all available CSF samples and genomic analyses of pretherapeutic PCNSL tissue biopsies are ongoing.
At the time of analysis, the median follow-up of the PCNSL cohort was 17 months (range: 1-47) with three deaths observed at 1, 2 and 4 months, respectively. Among the remaining eight patients, six are in continuous 1st complete remission (CR) and two in 2nd CR. Of the six patients in 1st CR, four underwent consolidation with upfront autologous transplant. So far, no significant correlation between CSF sPD-1 and survival has been observed.
Despite the limited size of our cohort, we demonstrated that sPD-1 is present and measurable in the CSF of previously untreated PCNSL patients. Moreover, we observed that sPD-1 levels in the pretherapeutic CSF samples from PCNSL were significantly higher than those measured in the non-PCNSL and neuro-screen samples. The PCNSL patients with the most adverse histological features were also the ones with the highest sPD-1 levels in the pretherapeutic CSF. Confirmation of these findings in a larger independent cohort as well as sequential measurements obtained during the course of the disease are warranted. If confirmed, our observations may help to evaluate the usefulness of CSF sPD-1 measurements with regard to disease monitoring as well as checkpoint inhibition strategies in PCNSL.
Pulczynski: Investigator of a clinical trial (Nordic Lymphoma Group NLG- LBC7(Polar Bear) EudraCT No 2018- 0038 funded by Roche: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal